

Treatment-naïve patients can
start and stay with Elfabrio1
The safety and efficacy of Elfabrio were studied in a treatment-naïve patient population or patients not treated in the past 26 weeks in NAÏVE.1,2
Open-label | Safety study
- Primary study: 3-month dose-ranging period (n=18)
- Extension study: 9 months (n=16)
- Open-label extension (1 mg/kg dose only; n=15): 60 months; up to 72 months of total treatment
Safety endpoints1,3
- Treatment-emergent adverse events (TEAEs), infusion-related reactions (IRRs), anti-drug antibodies (ADAs)
Other endpoints1,3,4
- Plasma lyso-Gb3, estimated glomerular filtration rate (eGFR), cardiac disease involvement, urine protein-to-creatinine ratio, pain
In the Elfabrio Prescribing Information, NAÏVE is referred to as Trial 1.
lyso-Gb3, globotriaosylsphingosine.
Tolerability and low immunogenicity were observed in treatment-naïve patients through a 5-year study1
Results at 1 year demonstrate:
Most adverse events were mild to moderate in treatment-naïve patients taking Elfabrio5
- The most common related TEAEs were nausea (25%), chest discomfort, dizziness, rash, and fatigue (13% each)
- 4 severe TEAEs were reported, with migraine and anaphylaxis considered to be possibly related and definitely related to Elfabrio treatment, respectively. Anaphylaxis was IgE-mediated hypersensitivity-related bronchospasm that led to study withdrawal
28.5% of Elfabrio-treated patients (4/14) who were ADA negative at baseline became ADA positive during Elfabrio treatment2*
| * | The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. |
| IgE, immunoglobulin E. | |
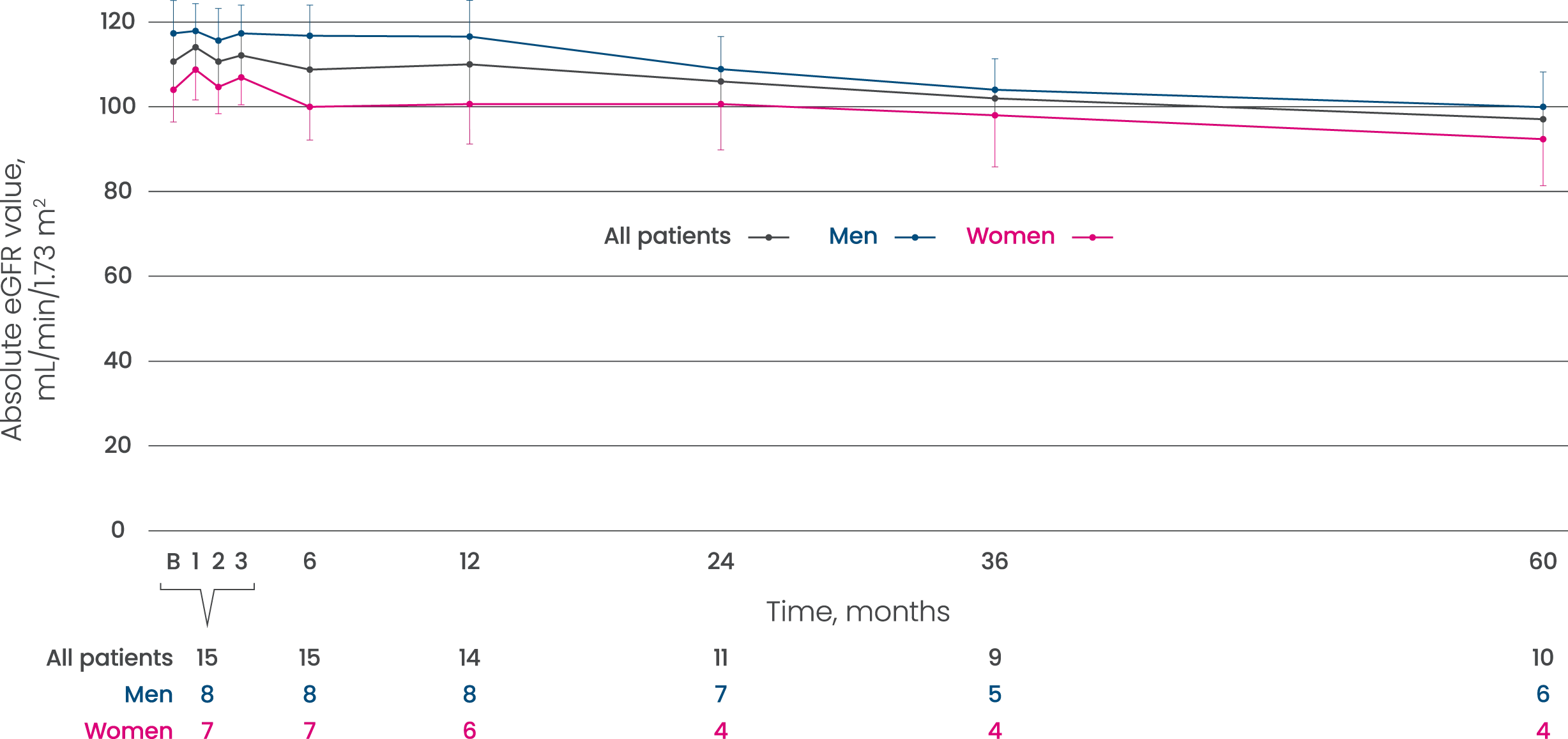
Elfabrio helped to maintain renal function (eGFR) over 60 months1
| Mean absolute eGFR for patients receiving Elfabrio over 60 months1 |
Consider Elfabrio to treat patients
for the long term.1
B, baseline.
Already considering patients for Elfabrio?








